top of page
BLOG


Metrics matter in aligning the organisation health care.
Which key performance indicators do YOU use to fight the pandemic in your health care system? Interested to discuss relevant metrics?...
Mar 16, 2021


Different attitudes generate different results:
All want to achieve herd immunity fast...but not all have the same attitudes...or generate the same results. Interested to discuss "can...
Mar 11, 2021


What miracles look like...
Interested to discuss Covid-19 project experience in Europe? Get in touch here!
Feb 18, 2021


Feb 12, 2021


Learning from Covid-19 projects: the only measure that is 100% effective against Covid-19:
Interested in more? Contact us!
Feb 4, 2021


SOP (II): Standard Operating Procedures & Technical Work Instructions:
The framework of governance & processes SOPs describe ways of working in a generic & binding way. Technical Work Instructions detail work...
Nov 3, 2020


SOP (I): Standard Operating Procedures:
The back-bone of compliant operations delivering safe & effective medicines to patients… ...and verifiable business processes to...
Oct 30, 2020


Webinars work - and so does working in webinars!
2 days learning and working together in teams from Nepal to Mexico and Scotland to Dubai come to an end. Looking forward to more online...
Sep 23, 2020


Vision & mission matter (I)
Where do you want to go? If you don't want to be living somebody else's future - choose your's!
Sep 11, 2020


Company culture matters (3): Measuring the drivers of performance helps...
...to establish a company culture of excellence
Sep 2, 2020


Company culture matters (2): Creating a competitive advantage...
...through measuring attitudes and beliefs that drive cultural excellence Interested in more? Contact us!
Aug 5, 2020


Company culture matters (1): Best practice in Post Merger Integration:
Measuring cultural change, drives new levels of performance Interested in more? Contact us!
Aug 4, 2020


HTA considerations II: regulators, HTA bodies and payers: the earlier - the better
Interested in more? Contact us!
Jul 15, 2020


HTA considerations I: pharma industry: the earlier - the better
Interested in more? Contact us!
Jul 15, 2020

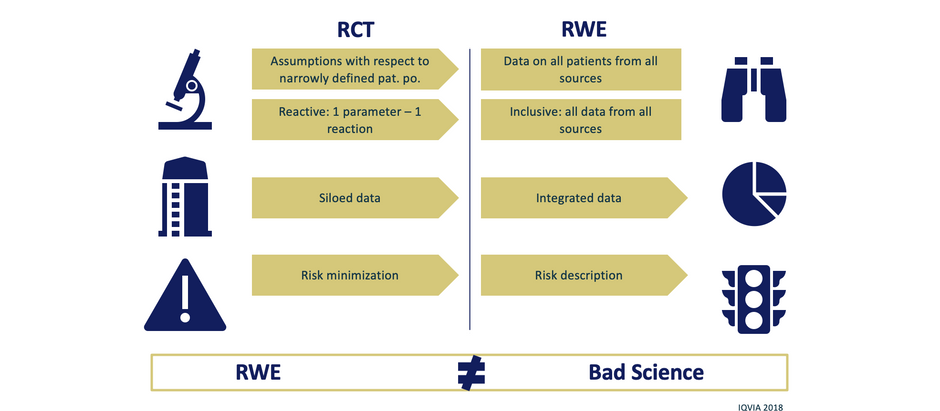
Randomized controlled trials are the shell of the evidence construction...
...and systematically Real-World Evidence data are the fittings Interested in more? Contact us!
Jul 1, 2020


How Real-World Evidence augments randomized controlled trials...
...to describe pharmaceutical value propositions: the more data, the better! Interested in more? Contact us!
Jul 1, 2020


Effective distributor management requires clear definitions of R&Rs
Interested in more? Contact us!
Jun 4, 2020


Virtual offices also have a floor-plan!
We help your organisation communicate more effectively for better customer results. Interested in more? Contact us!
May 14, 2020
bottom of page




